Artigo
| Application of TiO2 semiconductors for the simultaneous degradation of Naproxen, Diclofenac, and Mefenamic acid in surface waters |
|
José F. P. DinizI,* I. Programa de Pós-Graduação em Química, Instituto Federal de Educação, Ciência e Tecnologia do Maranhão, 65030-005 São Luís - MA, Brasil Received: 03/10/2025 *e-mail: prof.franciscodiniz@gmail.com The presence of contaminants, especially pharmaceuticals, in concentrations ranging from ng L-1 to μg L-1 has been the focus of numerous studies and is considered an emerging concern due to their adverse effects on aquatic biota and even human health. In this context, this study aimed to optimize a photoreactor equipped with four 24 W ultraviolet (UV)-A lamps and thin films of silver-doped titanium dioxide (Ag/TiO2), evaluating parameters such as catalyst concentration, pharmaceutical concentration, matrix effects, and pharmaceutical quantification using high-performance liquid chromatography (HPLC). The immobilized Ag/TiO2 catalysts were used for the photodegradation of the pharmaceuticals naproxen, diclofenac, and mefenamic acid. Photocatalysis tests were initially conducted in ultrapure water, followed by dispersive liquid-liquid microextraction with solidification of floating organic drops (DLLME-SFOD) on a real sample from São José Bay (Maranhão, Brazil) to identify the pharmaceuticals present and evaluate the degradation process. Naproxen and diclofenac were detected in the environmental sample, and results showed that photolysis alone was insufficient to degrade the compounds in brackish water. Therefore, photocatalytic treatment using
Ag/TiO2 was necessary, achieving 100% degradation of diclofenac in 7 h, 100% of mefenamic acid in 4 h, and 90% of naproxen in 10 h. This research highlights the effectiveness of the optimized photoreactor with Ag/TiO2 thin films in treating real matrices to achieve high drug degradation rates. INTRODUCTION Advances in drug development have improved the quality of life for both humans and animals, increasing average life expectancy. In this context, there has been a rise in the production of pesticides, medications, and personal care products. Consequently, the improper disposal of these products into the environment has become increasingly common.1 According to Patel et al.,2 pharmaceuticals have been deposited in aquatic environments since the last few decades and have emerged as a significant environmental threat. Studies show that substantial amounts of organic pollutants are found in surface waters worldwide.3 Generally, industrial wastewater treatment plants (IWWTP) and industrial effluents are the main point sources of water pollution.4 Substances known as emerging contaminants (ECs) are related to chemical compounds present in our daily lives, such as pharmaceuticals, hygiene products, veterinary products, food packaging, agrochemicals, flame retardants, and female sex hormones, among others. They are found in environmental and biological matrices at very low concentrations, on the order of ng L-1.5 Emerging contaminants are also referred to as emerging pollutants because they are not monitored or do not yet have regulations, as these substances can often affect the endocrine system and pose potential risks to health.6 One class of compounds that deserves special attention is non-steroidal anti-inflammatory drugs (NSAIDs), such as naproxen, ibuprofen, sodium diclofenac, and mefenamic acid. These drugs are recommended by healthcare professionals worldwide and are primarily used for the treatment of inflammation, pain, and swelling, osteoarthritis, rheumatoid arthritis, and musculoskeletal injuries.7,8 Several studies report the presence of these pharmaceuticals in wastewater and various aqueous matrices due to their persistence even after primary and secondary treatment. Among these studies is that of Tauxe-Wuersch et al.,9 who collected eighty-six wastewater samples in April 2002 at the Mittleres Emmental wastewater treatment plant in Hasle (Bern, central Switzerland), in January 2003 at the Lausanne wastewater treatment plant, and in February and June 2003, and January 2004 at the Morges wastewater treatment plant (Western Switzerland, on Lake Geneva). The authors found that none of the studied wastewater treatment plants were able to remove diclofenac from the wastewater, while the Mittleres Emmental facility removed half (28-74%) of mefenamic acid. Nieto et al.,10 observed that diclofenac is toxic to the crustacean Atyaephyra desmaresti; another question is how harmful ibuprofen and carbamazepine are to this crustacean. It was also noted that increasing temperatures can maximize toxicity for aquatic organisms. Naproxen has a half-life of 27 days, making it considered non-degradable and thus a persistent medication in the environment.11 Naproxen adversely affects aquatic organisms by incorporating residual concentrations within them. This medication can accumulate in the bile of fish, disrupting thyroid function and growth in zebrafish.12 In zebrafish, naproxen has also caused gastrointestinal problems, renal lesions, and hepatotoxicity. This occurs because this class of pharmaceuticals is not limited to human applications but is also prescribed in veterinary medicine and can be introduced into natural aquatic environments either metabolized or unchanged through domestic wastewater.13,14 Pharmaceuticals have been widely detected in groundwater, drinking water, and drinking water treatment plants, raising concerns about their effects on human health.15-17 This has become an emerging concern, as the occurrence, transport, fate, and adverse effects on aquatic biota and human health are still poorly understood.18 Concentrations in drinking water are much lower, reported in the ng L-1 range, which is significantly below their therapeutic doses. Therefore, daily doses at these levels are considered harmless; however, the long-term effects of such doses remain unknown.2 There are various methods in the literature for determining the presence of these pharmaceuticals. For example, Silva et al.19 proposed a methodology involving liquid-liquid microextraction using solidified floating organic drop microextraction (SFODME), which was combined with liquid chromatography and ultraviolet-visible (UV-Vis) detection to determine non-steroidal anti-inflammatory drugs (NSAIDs) such as naproxen (NPX), diclofenac (DCF), and mefenamic acid (MFN) in samples of tap water, surface water, and seawater. For environmental remediation, various studies have utilized silver (Ag)-doped titanium dioxide (TiO2) semiconductor due to its low cost and well-established antibacterial properties.20 This approach is also favored because the surface plasmon resonance (SPR) effect enhances the optical absorption of TiO2 in the visible region and reduces the recombination rate of photogenerated charge carriers through redox reactions at the metal-semiconductor interface.21,22 In the study by Jandaghian et al.,23 the degradation of the antibiotic levofloxacin under visible light was reported with a degradation rate of 49% using Ag/TiO2 and 22% with pure TiO2. Additionally, Urda et al.24 evaluated the degradation of the antibiotic sulfamethoxazole, observing a removal percentage of 90% under sunlight. Meirelles25 prepared TiO2-H2O2 and demonstrated its photocatalytic efficiency in degrading paracetamol, achieving removal rates between 92 and 98%. Almeida26 utilized TiO2 modified with malonic acid for the photodegradation of tetracycline and chlorophenol under low-power visible light (26 W), achieving extremely high photocatalytic activity, with approximately 100% degradation after 6 h. As a result, Ag/TiO2 photocatalysts have proven to be effective in degrading various emerging contaminants.27,28 Therefore, in this research, the extraction of non-steroidal anti-inflammatory drugs (NSAIDs) was conducted on surface waters collected from Baía de São José, MA, Brazil, using the liquid-liquid microextraction method with solidified floating organic drop. Subsequently, the samples were analyzed using high-performance liquid chromatography (HPLC). This technique was also employed to determine the concentrations and calculate the degradation rates of this group of pharmaceuticals, which includes diclofenac, naproxen, and mefenamic acid. Additionally, heterogeneous photocatalysis treatment was utilized, employing TiO2 catalysts obtained via the sol-gel method for the photodegradation of these compounds in the aquatic matrix. In this work, heterogeneous photocatalysis with TiO2 was performed, which is considered a promising semiconductor that has been studied for environmental remediation applications. This underscores the breadth of the topic and the possibilities for its exploration. Photocatalytic processes are reported as an effective means for treating emerging contaminants through redox reactions, generating less harmful transformation (degradation) products and, in some cases, achieving mineralization.29,30
EXPERIMENTAL Equipment For the studies on photodegradation, a bench-scale photoreactor was used, consisting of four UV lamps (λ = 365 nm) of 26 W each (Taschibra), a magnetic stirrer (Fisatom 751), and an air compressor (Vigor Ar 60). For the preparation of standard solutions, an analytical balance (model M214Ai, BEL Engineering) was employed, along with an ultrasonic bath (UNIQUE USC-1400), a pH meter (multiparameter meter edge®, Hanna), and a Millipore Direct 8 water purification system from Millipore. The efficiency of heterogeneous photocatalysis in degrading the studied pharmaceuticals was monitored using HPLC-UV-Vis, model LC 20AT prominence (Shimadzu®), equipped with an injector with a capacity of 20 µL and two high-pressure pumps coupled to two series detectors: one fluorescence detector (FD), model RF-10AXL, and another UV-Vis detector, model SPD-20A. A C-18 reversed-phase column packed with 5 µm diameter particles, model Luna from Phenomenex®, with a length of 250 mm and an internal diameter of 4.6 mm, was used. Data acquisition was performed using the LC Solution software, version 1.24 SP1 (Shimadzu Corporation, Japan, 2017). Analytical methodology Standards solutions, solvents and reagents The laboratory glassware used in the preparation of the solutions was previously washed with a 5% aqueous solution of Extran® neutral detergent and then rinsed with potable water and ultrapure water and conditioned with the appropriate solvent. The analytical standards of the anti-inflammatory drugs naproxen, sodium diclofenac, and mefenamic acid, all with a purity greater than 98%, were obtained from Sigma-Aldrich®. The dispersive solvents methanol and acetonitrile, both of chromatographic grade, were sourced from Merck, and ortho-phosphoric acid (85%) was obtained from Isofar. Catalyst used in heterogeneous photocatalysis treatments For this study, the catalyst Ag/TiO2 was developed using the sol-gel technique, which involves the preparation of a solution referred to as solution A. This solution results from the complexation of titanium isopropoxide (Ti(OC3H7)4) with acetic acid (molar ratio AcOH/Ti(OC3H7)4 = 4), followed by the addition of isopropanol (volume ratio C3H8O/Ti(OC3H7)4 = 1). Subsequently, solution B was prepared, consisting of a mixture of ethanol and HNO3 (molar ratios C2H6O/Ti(OC3H7)4 = 7.7 and HNO3/Ti(OC3H7)4 = 0.5). Silver nitrate was added to solution B at a concentration of 0.15% by mass of silver relative to TiO2. Additionally, TiO2 P25 was used in a proportion of 1.0 g of titanium per liter of ethanol, and this solution was sonicated for approximately 20 min before being mixed with solution A. After one hour of stirring, solution A was combined with solution B, resulting in a solution referred to as solution C, which was stirred for an additional 2 h. The immobilization in Pyrex glass tubes - using either three or five tubes with surface areas of 0.0132 and 0.0220 m2, respectively - was performed using the "dip-coating" method.31 The synthesis and immobilization processes for the films in the tubes were conducted following the methodology described by Araujo.31 Chromatographic conditions To identify the pharmaceuticals, present in the samples, a method validated by Silva et al.19 was employed, allowing for the quantification of diclofenac, naproxen, and mefenamic acid. The analytical conditions used were as follows: column [Luna C-18 (reversed phase), Phenomenex® (250 mm × 4.6 mm, 5 µm)], mobile phase [ACN/H3PO4 in water (pH 2.24) in a proportion of 60:40% (v/v)], solvent (methanol (MET) for stock and working solutions), flow rate (1.2 mL min-1), temperature (25 ºC), detector and wavelength (λ) [UV-Vis; 256 nm (0-7 min), 234 nm (7-15 min)], retention time (tR)(NPX) 5.5 min, tR (DCF) 8.8 min, tR (MFN) 13.0 min. Construction of analytical curves for NSAIDs Stock solutions at a concentration of 100 mg L-1 were prepared from the analytical standards of the pharmaceuticals by diluting each analyte in methanol. These solutions were kept refrigerated at a temperature of 4 ºC in amber glass.13,14 After defining the optimal chromatographic conditions to be employed during the analysis of the anti-inflammatory drugs, it became possible to construct the analytical curves for naproxen, diclofenac, and mefenamic acid at concentrations of 10, 25, 50, 100, 200, 300, 400, 500, and 600 µg L-1. This step was performed using nine working solutions prepared in methanol. Consequently, OriginLab, version 7.0 (OriginLab Corporation, USA, 2002) was used to construct the graphs and determine the equations of the lines and the coefficients of determination (R2). Photoreactor used in the study of drug degradation The photocatalytic tests, photolysis test, and dark control test were carried out in a photoreactor consisting of a rectangular box with two openings on the front and top, which can be fully closed. Its plywood walls are internally lined with aluminium foil, and it includes two removable wooden supports, each holding two lamp sockets. On the outside, there is a switch for the lamps located at the back of the reactor. Inside the box, four 25 W UV lamps were installed, each with maximum intensity at λ = 365 nm, along with a magnetic stirrer. The system also includes an aquarium pump to saturate the solution with atmospheric oxygen and a beaker containing the solution to be degraded. Photocatalytic treatment for NSAIDs and photolysis For the photolysis and photocatalysis treatments, 800 mL of solution was prepared with a concentration of 200 µg L-1 of the pharmaceuticals naproxen, diclofenac, and mefenamic acid. The photolysis test lasted 6 h, with samples collected at 2-h intervals. During the photocatalytic treatment, aliquots were taken and placed in 2 mL vials to be analyzed by HPLC-UV-Vis. A dark test was also performed, consisting of the photoreactor with the catalysts but without UV light, lasting 4 h. In the photocatalytic tests, 3 and 5 test tubes coated with Ag/TiO2 films immobilized on the outer surface were used to evaluate the influence of the catalyst area on photodegradation over 15 h. During this stage, aliquots were collected every hour. During the photocatalytic experiments to assess photodegradation efficiency, the data were analyzed based on aliquots taken at various times in the photoreactor and expressed as percentage removal, calculated according to Equation 1: 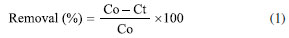 where: C0 is the initial concentration (µg L-1) of the pollutants after equilibrium and Ct is the concentration (µg L-1) of the pollutants after t (min) of irradiation. Sampling site The water samples were collected from São José Bay, MA (Figure 1S, Supplementary Material), located on the Eastern side of São Luís Island. This site has shallow depths and receives water from the Itapecuru and Munim Rivers.32 As part of the Maranhão Gulf, it is classified as a high-energy region and is constantly subjected to the combined effects of coastal currents generated by various hydrodynamic forces such as tides, waves, and trade winds, as well as the discharge of several rivers, forming a large estuarine complex.33 The seawater sample was collected on April 30, 2024, by Labciclos/Departament of Oceanography and Limnology (DEOLI)/Federal University of Maranhão (UFMA), at the coordinates: latitude -2.300561 N and longitude -44.123748 E.
RESULTS AND DISCUSSION Optimization of the chromatographic conditions for the pharmaceutical analysis Initially, three stock solutions were prepared with a concentration of 100 mg L-1 of NPX, DCF, and MFN from their standards. An analytical curve was constructed for each stock solution using nine different concentrations. The working linear range for the three pharmaceuticals was established with the following concentrations: 10, 25, 50, 100, 200, 300, 400, 500, and 600 µg L-1, analyzed by HPLC to generate the calibration curves. The calibration curves were obtained using working solutions prepared in methanol. Figure 2S (Supplementary Material) presents the parameters of the analytical curves for the NSAIDs NPX (Figure 2Sa), DCF (Figure 2Sb), and MFN (Figure 2Sc), with respective linear equations: y = 3.0942x - 7.4359, y = 3.1538x + 25.287, and y = 5.5303x + 63.072. The analytical curves for the NSAIDs were satisfactory considering their respective coefficients of determination (R2): MFN (0.9996), NPX (0.9998), and DCF (0.9999), indicating good linearity and compliance with the guidelines for method validation.34,35 With these conditions, it was possible to determine the retention times for the pharmaceutical naproxen (5.2 min), diclofenac (8.9 min), and mefenamic acid (13.9 min), as well as their respective limits of detection (LOD): LODNPX = 2 µg L-1; LODDCF = 2 µg L-1; LODMFN = 4 µg L-1, and limits of quantification (LOQ): LOQNPX = 7 µg L-1; LOQDCF = 6 µg L-1; LOQMFN = 14 µg L-1. Verification of retention times of pharmaceuticals at pH 6 The optimized and validated chromatographic method by Silva et al.19 for NSAID analysis via HPLC was employed for studies on the drug extraction method at pH 2. In the analysis conducted in this work, the pH was not altered; that is, it was maintained at the value of Milli-Q water, pH 6. Due to this difference, analyses were performed using HPLC-UV-Vis at pH 6 to assess whether this variation in pH values would affect the chromatographic signals and retention times of the pharmaceuticals. The results showed that this pH variation in the solutions containing the pharmaceuticals did not produce significant changes in their retention times compared to the studies by Silva et al.,19 indicating that the chromatographic method used was effectively optimized for the studies throughout this work under the same conditions as those reported by the author. Study of adsorption on Ag/TiO2 films The dark test involves verifying the activity of the catalyst in the absence of irradiation, i.e., assessing whether the employed catalyst exhibits adsorptive properties for the studied compounds. In this experiment, initial concentrations of 200 μg L-1 for each of the three pharmaceuticals were used. The results shown in Figure 1 confirm that there was no significant change in the concentrations of NPX, DCF, and MFN over a period of 4 h.
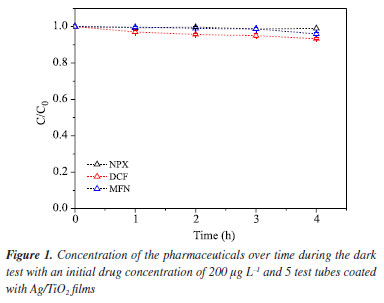
The results of the test show that 1 h is sufficient for the adsorption/desorption equilibrium of the pharmaceuticals on the catalyst to be established, which aligns with the findings of Ferreira,36 who observed this equilibrium after 30 min of contact between TiO2 catalyst in suspension with the pharmaceuticals. Influence of catalyst area on photocatalysis tests The influence of catalyst area was evaluated in heterogeneous photocatalysis experiments. Initially, three tubes with Ag/TiO2 films were used, covering a total catalyst area of 0.0132 m2. For the second test, five tubes were employed, resulting in an area of 0.0220 m2. Figure 3S (Supplementary Material) illustrates the degradation rate of the pharmaceuticals with the increase in catalyst area. The results showed that using five Ag/TiO2 films led to higher degradation percentages, approximately 24% for DCF and around 40% for MFN, compared to the degradation observed with three films. However, for NPX, increasing the film area did not significantly affect the degradation rate. The effect of increasing the catalyst area can also be investigated by evaluating the degradation kinetics. Literature37-40 indicates that the degradation reactions of NSAIDs typically follow first-order kinetics. This is demonstrated through plots of ln(C/C0) versus time (first-order kinetics), where C is the concentration at time t and C0 is the initial concentration. Using this approach, it was confirmed that the degradation follows first-order behavior, and the kinetic data are presented in Table 1.
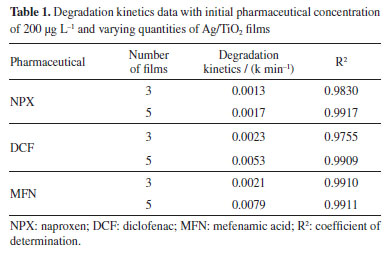
Therefore, by analyzing Figure 3S (Supplementary Material) and Table 1, it was observed that increasing the catalyst area for naproxen did not significantly enhance the reaction rate. In contrast, for diclofenac, the rate nearly doubled, and for mefenamic acid, it quadrupled. A similar result was obtained by Lima et al.,41 who used the same Ag/TiO2 films in their study, evaluating three different film areas: 0.0132, 0.0176, and 0.0220 m2. The results showed that the rate constants increased linearly with the increase in film area for various estrogens, reaching approximately 90% degradation of all three compounds. In summary, the behavior observed in this study for diclofenac was consistent with those findings. Influence of pharmaceutical concentration The pharmaceuticals concentrations studied were approximately 200 and 400 µg L-1, in order to evaluate the effect of catalysts at different concentrations. These concentrations are higher than those reported in other studies in the literature, such as Oliveira and co-authors,42 who confirmed a naproxen concentration of 75.90 µg L-1 in the Rio Monjolinho, SP, and Kramer et al.,43 who analyzed a diclofenac concentration of 0.285 µg L-1 in the Iguaçu River, Curitiba, PR, indicating higher levels in rivers. As for mefenamic acid, it was difficult to find data in the literature; despite numerous reports of its presence in domestic sewage, surface and groundwater, this compound is poorly soluble in water. Several authors44,45 have reported low solubility even under varying pH, temperature, and concentration conditions. The study of the influence of pharmaceutical concentration on the mixture showed that decreasing the diclofenac concentration positively affected its degradation, as observed in Figure 4S (Supplementary Material) and Table 2. However, for mefenamic acid and naproxen, increasing the concentration to double the initially tested amount resulted in almost no change in the reaction rate. Regarding naproxen, as expected, degradation remained low; after 7 h of treatment, only about 15% degradation was observed with doubling of the drug concentration. For diclofenac, this doubling caused a significant change: after 6 h, degradation was 90% at the lower concentration but only about 20% at an initial concentration of 400 µg L-1. This result can be attributed to the fact that at higher concentrations of compounds, there may be competition among transformation products within the reaction medium, slowing down the reaction rate.46
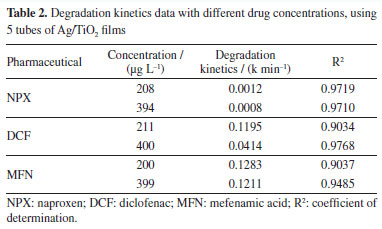
It can be observed in Table 2 that mefenamic acid exhibits almost the same reaction rate at both concentrations. The reaction rate of naproxen was nearly doubled at the lower concentration studied. The most significant result was observed with diclofenac, which showed an increase of up to three times in the value of the rate constant. Although these concentrations are still below those typically found in aquatic environments, these results demonstrate that Ag/TiO2 films are capable of degrading the pollutants studied. Physical-chemical and metal characterization of the real samples The seawater sample was collected on April 30, 2024, by Labciclos/DEOLI/UFMA. The quantification of water quality parameters; dissolved oxygen (DO), temperature, and pH, was performed in situ with the aid of a Hanna HI 98494 multiparameter probe. The following results were obtained: pH (7.35), Eh (oxidation-reduction potential (ORP), 53.5 mV), dissolved oxygen (DO) saturation (106.9%), salinity (5.42 PSU), temperature (28.42 ºC). According to National Council for the Environment (CONAMA) Resolution 357/2005,47 this water sample is classified as brackish water, indicated by the salinity concentration of 5.42 PSU, and categorized under water quality standards as class 1 brackish water, based on its pH, DO, and total dissolved solids (TDS) values. Such brackish waters can support fishing or cultivation of organisms for intensive consumption purposes. This sample has an Eh value (mV ORP) of 53.5, which refers to the oxidation-reduction potential measured in millivolts (mV). This value indicates the tendency of the solution to lose electrons, which is essential in chemical and biological processes. The positive Eh suggests an oxidizing environment. In brackish waters, mixtures of freshwater and seawater, the redox potential can vary due to chemical composition and interactions among ions present. The value of 53.5 suggests an environment conducive to certain biogeochemical reactions, influencing aquatic life and ecosystem health. Metal analysis was conducted at the Laboratory of Food, Beverage, and Environmental Analysis and Research at the Federal Institute of Maranhão, Monte Castelo Campus. The purpose was to identify the presence of metals Cu, Pb, Cd, Cr, and Ni in the sample to assess their activity and interference in photocatalytic processes. The analysis was performed using ICP-OES (inductively coupled plasma optical emission spectrometry) from PerkinElmer, model Optima 7000 DV. The working range was 5-1000 µg L-1; however, all metals were below the detection limit (LOD), and the concentrations observed are as follows: Cu (> LOD), Pb (> LOD), Cd (> LOD), Cr (> LOD), and Ni (> LOD). Determination of pharmaceuticals in the real sample using DLLME-SFOD In the laboratory, the water sample was subjected to the dispersive liquid-liquid microextraction with floating organic drop solidification (DLLME-SFOD) process, and subsequently analyzed using high-performance liquid chromatography (HPLC), following the method proposed by Silva et al.19 Diclofenac and naproxen were detected in the sample; however, it was not possible to observe an analytical signal for mefenamic acid. This occurs due to the low solubility of this pharmaceutical in water. Literature44,45 indicates that mefenamic acid tends to deposit at the bottom of seas, rivers, and oceans. Photolysis in purified water For the photolysis tests, the initial concentrations of the pharmaceuticals were 211 μg L-1 (NPX), 187.11 μg L-1 (DCF), and 202.34 μg L-1 (MFN). The results of this control test are shown in Figure 2, which presents the percentage of the pharmaceuticals concentrations remaining in the aqueous solution at different times during the photolysis treatment.
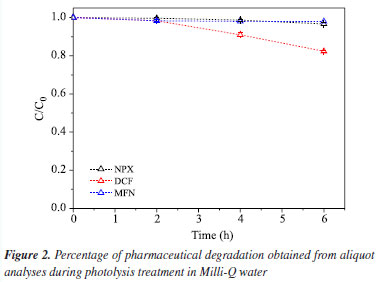
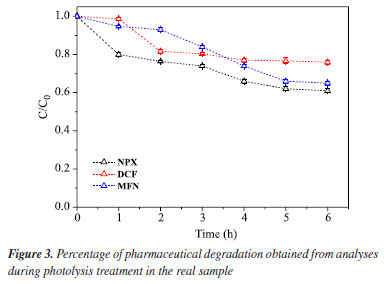
As can be seen in Figure 2, naproxen showed low degradation after 6 h of treatment (2.9%). A similar result was obtained by Paniagua et al.,48 who observed in their study that direct photolysis is not capable of degrading naproxen, thus requiring the use of another process as the main degradation pathway. The diclofenac compound achieved greater removal, especially during the first hour of treatment compared to the other compounds, totaling 18.2% at the end of the process. This confirms findings from various researchers,16,17 who have reported that diclofenac has a high rate of direct photolytic degradation, even under visible light in systems with Milli-Q water. Among the analyzed compounds, mefenamic acid exhibited the lowest degradation rate, reaching only 2% at the end of the process. This aligns with the study by Yamamoto et al.,49 who reported the persistence of this pharmaceutical after 72 h under visible light, with a degradation rate of approximately 20%, indicating its relative stability. Photolysis in real sample The photolysis of the real sample conducted over 6 h of treatment showed a linear trend starting from 2 h, with a degradation of 20% for DCF and 33% for NPX and MFN. This demonstrates the stability of these pharmaceuticals in the environment. In an oxidizing brackish water environment, photolysis can occur, but its efficiency may be affected by various factors such as the presence of salts, water turbidity, and organic matter content.50 Studies51,52 indicate that photolysis has potential as an alternative treatment method to remove various organic contaminants; however, one of its disadvantages is the formation of harmful intermediates, which can make this type of treatment unviable.53 In Figure 3, shows that diclofenac under photolysis in seawater reaches a degradation stabilization at 20% after just 2 h of treatment, confirming the strong influence of the matrix on the degradation of this pharmaceutical. However, mefenamic acid in pure water exhibited a very low degradation rate; in seawater, this rate increased, with the process ending after 6 h with 33.7% of the compound degraded.
Photocatalysis by UV irradiation of pharmaceuticals using 5 tubes immobilized with Ag/TiO2 films in purified water and real samples Photocatalysis in purified water Considering the preliminary tests, adsorption, photolysis, and catalyst area evaluation, photocatalytic experiments were conducted under the best conditions obtained in the experiments with Milli-Q water. The test began with 30 min of agitation to establish the adsorption/desorption equilibrium, followed by a 16-h photocatalytic treatment under UV-Vis light. The system employed a catalyst with a surface area of 0.0220 m2, corresponding to five Ag/TiO2 films, and an initial concentration of 200 μg L-1 for each of the three pharmaceuticals, as shown in Figure 4.
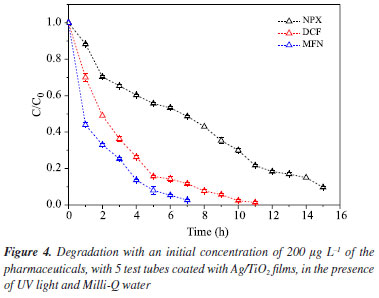
In the photocatalysis test, a radical preference for the mefenamic acid compound was observed, which was completely degraded after 7 h. This result is similar to that found in another study37 using higher concentrations of mefenamic acid (2 mg L-1) with irradiation employing a 500 W mercury lamp for 5 h of treatment. Diclofenac, as the second preferred radical, was fully degraded after 10 h of irradiation, while naproxen showed the lowest radical preference; thus, at the end of the experiment, 90% of the compound was degraded. These results from heterogeneous photocatalysis (HP), using Ag/TiO2 films, demonstrated to be a more suitable treatment compared to photolysis, as HP increased the photodegradation rate for all three pharmaceuticals. In photolysis, after 16 h, only 30.8% of NPX was degraded, whereas in photocatalytic treatment over 15 h, 90% of the drug was degraded. Regarding diclofenac in the control test, only 62.5% degradation occurred after 16 h, while with HP in just 11 h, there was no detectable analytical signal of the compound. For mefenamic acid, better performance was observed: photolysis had only degraded about 9% after 16 h, whereas with heterogeneous photocatalysis, complete photodegradation of the compound was achieved after just 7 h. TiO2 catalysts are already known for their excellent performance in numerous studies41,54-57 where pharmaceuticals were treated via photocatalysis; and the catalyst used in this study is doped with silver, which delays electron-hole recombination;58 increasing the removal capacity of the studied pollutants even in a real matrix composed of various substances and ions. Photocatalysis in real sample Photocatalysis was performed under the best conditions identified during the research (5 films of Ag/TiO2, in the presence of UV light, with aeration). To evaluate the photocatalytic degradation activity, it was necessary to add the pharmaceuticals, fortifying the sample with 200 μg L-1 of each substance. In Figure 5, it can be observed that the radical preference remains in the following order: mefenamic acid, diclofenac, and naproxen in the real sample, as well as in tests with pharmaceuticals in Milli-Q water.
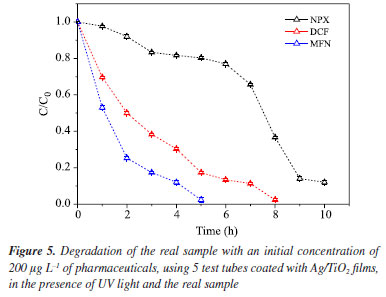
The results obtained were satisfactory; a reduction in degradation time by photocatalysis for all three pharmaceuticals was observed. For example, naproxen degraded 89% in 10 h, whereas this level of degradation in pure water only occurred after 15 h of drug exposure. Diclofenac degraded 88% in 7 h, and after 8 h no signal of this compound was detected - an important time saving compared to pure water, which degraded completely in 11 h. Similarly, mefenamic acid had its last detectable concentration at 4 h with a 90% degradation; no analytical signal was detected at 5 h. Compared to pure water, where complete degradation occurred in 7 h, there was also a reduction in degradation time from 7 to 4 h. This result is of great importance, as it demonstrates that even in a matrix as complex as seawater, it is possible to achieve satisfactory degradation of these compounds, and even more rapidly than in pure water. Among the parameters that may have contributed to this enhanced photocatalytic activity in seawater are the high amount of dissolved oxygen and the fact that it is a sample with oxidative potential.
CONCLUSIONS In the tests that evaluate the increase in catalyst surface area, it was evident that the use of 5 immobilized films of Ag/TiO2, totalling 0.0220 m2 of area, yielded the best results in degrading the three pharmaceuticals. Comparing the action of the catalyst on the three pharmaceuticals in this study, it is possible to conclude that there is a radical preference for mefenamic acid, which after 7 h of degradation showed no further indication of the compound in the analysis performed by HPLC. Diclofenac degrades much more effectively at lower concentrations, specifically at 200 μg L-1 as used in this study. Meanwhile, naproxen and mefenamic acid exhibited similar behavior when degraded at both concentration ranges studied: 200 and 400 μg L-1. Mefenamic acid is easily degraded with a catalyst surface area of 0.0220 m2, which quadrupled its reaction rate compared to a surface area of 0.0132 m2. For the other two pharmaceuticals, the amount of catalyst did not significantly influence the reaction rate. Photolysis tests on real samples, although showing better results than in pure water, are still insufficient to fully degrade these compounds in an aqueous medium. After several hours of photolysis, stability was observed around 40% degradation for naproxen and mefenamic acid, and about 20% for diclofenac. Therefore, it is necessary to employ photocatalytic treatment using the Ag/TiO2 catalyst; with this approach, it was possible to degrade diclofenac in 7 h, mefenamic acid in 4 h, and achieve 90% degradation of naproxen in 10 h in the real sample.
SUPPLEMENTARY MATERIAL The supplementary material for this work (images of the sampling point and the analytical curves for the three pharmaceuticals, as well as the influence of the catalyst surface area and the influence of pharmaceutical concentration) is available as a PDF file with free access at http://quimicanova.sbq.org.br/.
DATA AVAILABILITY STATEMENT The authors declare that all data is fully accessible within the text.
ACKNOWLEDGMENTS To the Instituto Federal de Educação, Ciência e Tecnologia do Maranhão (IFMA) for the infrastructure, and to its Graduate Program in Chemistry for the technical and scientific support.
AUTHOR CONTRIBUTIONS José F. P. Diniz was responsible for writing (original draft, review and editing), visualization, validation, methodology, investigation, formal analysis, conceptualization; Sana K. P. Viana for methodology, investigation, formal analysis, conceptualization; Gilmar S. da Silva for writing (review and editing), visualization, validation, methodology, investigation, formal analysis, conceptualization; Lanna K. Silva for validation, methodology, formal analysis; Samara A. Eschrique for writing (review and editing), visualization; Adriana B. Araújo for writing (review and editing), visualization, project administration, fundraising.
REFERENCES 1. Starling, M. C. V. M.; Amorim, C. C.; Leão, M. M. D.; J. Hazard. Mater. 2019, 372, 17. [Crossref] 2. Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C. U.; Mohan, D.; Chem. Rev. 2019, 119, 3510. [Crossref] 3. Tousova, Z.; Oswald, P.; Slobodnik, J.; Blaha, L.; Muz, M.; Hu, M.; Brack, W.; Krauss, M.; Di Paolo, C.; Tarcai, Z.; Seiler, T. B.; Hollert, H.; Koprivica, S.; Ahel, M.; Schollée, J. E.; Hollender, J.; Suter, M. J. F.; Hidasi, A. O.; Schirmer, K.; Sonavane, M.; Ait-Aissa, S.; Creusot, N.; Brion, F.; Froment, J.; Almeida, A. C.; Thomas, K.; Tollefsen, K. E.; Tufi, S.; Ouyang, X.; Leonards, P.; Lamoree, M.; Torrens, V. O.; Kolkman, A.; Schriks, M.; Spirhanzlova, P.; Tindall, A.; Schulze, T.; Sci. Total Environ. 2017, 601-602, 1849. [Crossref] 4. Petrie, B.; Barden, R.; Kasprzyk-Hordern, B.; Water Res. 2015, 72, 3. [Crossref] 5. de Aquino, S. F.; Brandt, E. M. F.; Chernicharo, C. A. L.; Eng. Sanit. Ambiental 2013, 18, 187. [Crossref] 6. la Farré, M.; Pérez, S.; Kantiani, L.; Barceló, D.; TrAC, Trends Anal. Chem. 2008, 27, 991. [Crossref] 7. Batlouni, M.; Brazilian Archives of Cardiology 2010, 94, 556. [Crossref] 8. Toledo-Neira, C.; Álvarez-Lueje, A.; Talanta 2015, 134, 619. [Crossref] 9. Tauxe-Wuersch, A.; de Alencastro, L. F.; Grandjean, D.; Tarradellas, J.; Water Res. 2005, 39, 1761. [Crossref] 10. Nieto, E.; Hampel, M.; González-Ortegón, E.; Drake, P.; Blasco, J.; J. Hazard. Mater. 2016, 313, 159. [Crossref] 11. Li, Q.; Wang, P.; Chen, L.; Gao, H.; Wu, L.; Environ. Sci. Pollut. Res. 2016, 23, 18832. [Crossref] 12. Xu, C.; Niu, L.; Guo, H.; Sun, X.; Chen, L.; Tu, W.; Dai, Q.; Ye, J.; Liu, W.; Liu, J.; Sci. Total Environ. 2019, 676, 387. [Crossref] 13. Beldean-Galea, M. S.; Coman, V.; Thiébaut, D.; Vial, J.; J. Sep. Sci. 2015, 38, 641. [Crossref] 14. Shukri, D. S. M.; Sanagi, M. M.; Ibrahim, W. A. W.; Abidin, N. N. Z.; Aboul-Enein, H. Y.; Chromatographia 2015, 15-16, 987. [Crossref] 15. Fick, J.; Söderström, H.; Lindberg, R. H.; Phan, C.; Tysklind, M.; Larsson, D. G. J.; Environ. Toxicol. Chem. 2009, 28, 2522. [Crossref] 16. Heberer, T.; Toxicol. Lett. 2002, 131, 5. [Crossref] 17. Kim, I.; Yamashita, N.; Tanaka, H.; J. Hazard. Mater. 2009, 166, 1134. [Crossref] 18. De la Obra, I.; Ponce-Robles, L.; Miralles-Cuevas, S.; Oller, I.; Malato, S.; Sánchez Pérez, J. A.; Catal. Today 2017, 287, 10. [Crossref] 19. Silva, L. K.; Rangel, J. H. G.; Brito, N. M.; Sousa, E. R.; Sousa, E. M. L.; Lima, D. L. D.; Esteves, V. I.; Freitas, A. S.; Silva, G. S.; Anal. Bioanal. Chem. 2021, 413, 1851. [Crossref] 20. Soo, J. Z.; Chai, L. C.; Ang, B. C.; Ong, B. H.; ACS Appl. Nano Mater. 2020, 3, 5743. [Crossref] 21. Ibukun, O.; Jeong, H. K.; Current Applied Physics 2020, 20, 23. [Crossref] 22. Al-Arfaj, E. A.; Superlattices Microstruct. 2013, 62, 285. [Crossref] 23. Jandaghian, F.; Pirbazari, A. E.; Tavakoli, O.; Asasian-Kolur, N.; Sharifian, S.; J. Hazard. Mater.Adv. 2023, 9, 100240. [Crossref] 24. Urda, A.; Radu, T.; Socaci, C.; Floare-Avram, V.; Cosma, D.; Rosu, M. C.; Pogacean, F.; J. Photochem. Photobiol., A 2022, 425, 113701. [Crossref] 25. Meirelles, M. R.: Materiais à Base de Carvão Ativado Proveniente de Resíduos Lignocelulósicos para Suporte de Fotocatalisadores com Aplicação em Remediação Ambiental; MSc Dissertation, Universidade Federal de São Paulo, São Paulo, Brasil 2024. [Link] accessed in July 2025 26. Almeida, L. A. L.: Complexos de Transferência de Carga com Alta Área Superficial Baseados em TiO2 Nanométrico Modificado com Ligantes Bidentados: Síntese, Caracterização e Atividade Fotocatalítica sob Luz Visível de Baixa Potência; PhD Thesis, Pontífica Universidade Católica do Rio de Janeiro, Rio de Janeiro, Brasil 2023. [Link] accessed in July 2025 27. Feng, Y.; Rijnaarts, H. H.; Yntema, D.; Gong, Z.; Dionysiou, D. D.; Cao, Z.; Wang, Y.; Miao, S.; Ye, Y.; Water Res. 2020, 186, 116327. [Crossref] 28. Zarzzeka, C.; Goldoni, J.; de Oliveira, J. R. P.; Lenzi, G. G.; Bagatini, M. D.; Colpini, L. M. S.; Sustainable Chemistry for the Environment 2024, 8, 100177. [Crossref] 29. Schneider, M. V.; Rosa, M. F.; Lobo, V. S.; Bariccatti, R. A.; Eng. Sanit. Ambiental 2014, 19, 61. [Crossref] 30. de Araújo, K. S.; Antonelli, R.; Gaydeczka, B.; Granato, A. C.; Malpass, G. R. P.; Rev. Ambiente Agua 2016, 11, 387. [Crossref] 31. Araujo, A. B: Degradação de Poluentes Orgânicos Utilizando Filmes de TiO2 Modificados com Íons Prata; PhD Thesis, Universidade Estadual Paulista, Araraquara, Brasil, 2006. [Link] accessed in July 2025 32. Silva, M. H. L.; Torres Junior, A. R.; Castro, A. C. L.; Azevedo, J. W. J.; Ferreira, C. F. C.; Cardoso, R. L.; Nunes, J. L. S.; Carvalho-Neta, R. N. F.; Iheringia, Série Zoologia 2018, 108, e2018018. [Crossref] 33. Silva, M. H. L.; de Castro, A. C. L.; da Silva, I. S.; Cabral, P. F. P.; Azevedo, J. W. J.; Soares, L. S.; Bandeira, A. M.; Basso, M. J.; Nunes, J. L. S.; Mar. Pollut. Bull. 2023, 186, 114477. [Crossref] 34. Ribani, M.; Bottoli, C. B. G.; Collins, C. H.; Jardim, I. C. S. F.; Melo, L. F. C.; Quim. Nova 2004, 27, 771. [Crossref] 35. Agência Nacional de Vigilância Sanitária (ANVISA); Resolução da Diretoria Colegiada (RDC) No. 166, de 24 de julho de 2017, Dispõe sobre a Validação de MétodosAnalíticos e dáOutras Providências; Diário Oficial da União (DOU), Brasília, No. 141, de 25/07/2017, p. 87. [Link] accessed in July 2025 36. Ferreira, I. V.: Modificação de Superfícies e Imobilização de Nanopartículas Semicondutoras para o Desenvolvimento de Novos Materiais Fotocatalíticos; MSc Dissertation, Universidade de Lisboa, Lisboa, Portugal, 2021. [Link] accessed in July 2025 37. Chen, P.; Wang, F. L.; Yao, K.; Ma, J. S.; Li, F. H.; Lv, W. Y.; Liu, G. G.; Bull. Environ. Contam. Toxicol. 2016, 96, 203. [Crossref] 38. Werner, J. J.; McNeill, K.; Arnold, W. A.; Chemosphere 2005, 58, 1339. [Crossref] 39. Kanakaraju, D.; Motti, C. A.; Glass, B. D.; Oelgemöller, M.; Environ. Sci. Pollut. Res. 2016, 23, 17437. [Crossref] 40. Kanakaraju, D.; Motti, C. A.; Glass, B. D.; Oelgemöller, M.; Chemosphere 2015, 139, 579. [Crossref] 41. Lima, K. V.; Emídio, E. S.; Nogueira, R. F. P.; Vasconcelos, N. S. L.; Araújo, A. B.; J. Chem. Technol. Biotechnol. 2020, 95, 2656. [Crossref] 42. Almeida, E. S.; de Oliveira, D.; Hotza, D.; Bioprocess Biosyst. Eng. 2017, 40, 1291. [Crossref] 43. Kramer, R.; Mizukawa, A.; Ide, A.; Marcante, L.; Santos, M.; Azevedo, J.; Revista Brasileira de Recursos Hídricos 2015, 20, 657. [Crossref] 44. Patil, P.; Gupta, V.; Udupi, R.; Srikanth, K.; Prasad, B.; Res. J. Pharm., Biol. Chem. Sci. 2010, 1, 544. [Link] accessed in July 2025 45. Moruzzi, R. B.; Lima, V. B.; Colombo, R.; Conceição, F. T.; Lanza, M. R. V.; Quim. Nova 2014, 37, 1594. [Crossref] 46. Malato, S.; Fernández-Ibáñez, P.; Maldonado, M. I.; Blanco, J.; Gernjak, W.; Catal. Today 2009, 147, 1. [Crossref] 47. Conselho Nacional do Meio Ambiente (CONAMA); Resolução No. 357, de 17 de março de 2005, Dispõe sobre a Classificação dos Corpos de Água e Diretrizes Ambientais para o seu Enquadramento, bem como Estabelece as Condições e Padrões de Lançamento de Efluentes, e dá Outras Providências; Diário Oficial da União (DOU), Brasília, No. 053, de 18/03/2005. [Link] accessed in July 2025 48. Paniagua, C. E. S.; Amildon Ricardo, I.; Marson, E. O.; Gonçalves, B. R.; Trovó, A. G.; J. Environ. Chem. Eng. 2019, 7, 103164. [Crossref] 49. Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Nakamura, Y.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J.; Water Res. 2009, 43, 351. [Crossref] 50. Oliveira, J. T.: Estudo Comparativo dos Processos de Eletrocoagulação e Fotólise com Aeração na Remoção de Fármacos em Efluentes de Estação de Tratamento de Esgoto (ETE); PhD Thesis, Universidade Federal do Ceará, Fortaleza, Brasil, 2024. [Link] accessed in July 2025 51. Li, F. H.; Yao, K.; Lv, W. Y.; Liu, G. G.; Chen, P.; Huang, H. P.; Kang, Y. P.; Bull. Environ. Contam. Toxicol. 2015, 94, 479. [Crossref] 52. Carlson, J. C.; Stefan, M. I.; Parnis, J. M.; Metcalfe, C. D.; Water Res. 2015, 84, 350. [Crossref] 53. da Silva, R. P.: Estudo da Fotodegradação em Águas: Avaliação da Degradação e Toxicidade; PhD Thesis, Universidade Federal da Paraíba, João Pessoa, Brasil, 2017. [Link] accessed July 2025 54. Yi, C.; Liao, Q.; Deng, W.; Huang, Y.; Mao, J.; Zhang, B.; Wu, G.; Sci. Total Environ. 2019, 684, 527. [Crossref] 55. He, Y.; Sutton, N. B.; Rijnaarts, H. H. H.; Langenhoff, A. A. M.; Appl. Catal., B 2016, 182, 132. [Crossref] 56. Asadi, A.; Akbarzadeh, R.; Eslami, A.; Jen, T. C.; Oviroh, P. O.; Energy Procedia 2019, 158, 4542. [Crossref] 57. Eslami, A.; Amini, M. M.; Asadi, A.; Safari, A. A.; Daglioglu, N.; Inorg. Chem. Commun. 2020, 115, 107888. [Crossref] 58. Pelaez, M.; Nolan, N. T.; Pillai, S. C.; Seery, M. K.; Falaras, P.; Kontos, A. G.; Dunlop, P. S. M.; Hamilton, J. W. J.; Byrne, J. A.; O'Shea, K.; Entezari, M. H.; Dionysiou, D. D.; Appl. Catal., B 2012, 125, 331. [Crossref]
Associate Editor handled this article: Cassiana C. Montagner |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Química Nova
Publicações da Sociedade Brasileira de Química
Caixa Postal: 26037
05513-970 São Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access






